Summary
- Share price has recently stumbled due to results of the leading vaccines.
- Vaccine development timeline is way behind the leading vaccines.
- Altimmune COVID-19 treatments have some unique and differentiated characteristics.
- Q1 brings with it two catalysts in the form of COVID-19-related data readouts.
Company
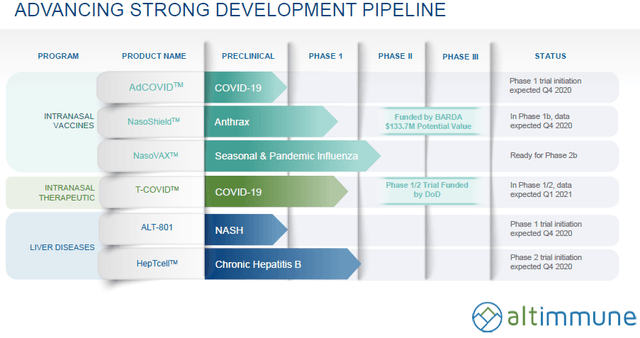
Altimmune, Inc. (NASDAQ:ALT) is a clinical stage biopharmaceutical company, focused on developing treatments for liver disease, immune modulating therapies, and vaccines. The company develops HepTcell, an immunotherapeutic product candidate that has completed Phase I clinical trial for patients chronically infected with the hepatitis B virus; NasoShield, an anthrax vaccine to provide for protection after a single intranasal administration and NasoVAX, a recombinant intranasal vaccine product candidate. Its preclinical stage products include ALT-801, a novel peptide-based dual GLP-1/Glucagon receptor agonist for the treatment of non-alcoholic steatohepatitis; and ALT-702, an investigational tumor immunostimulant for treating cancer. The company also develops veterinary product candidates.
Additionally, and most importantly, the company develops AdCOVID, a single-dose intranasal vaccine to protect against COVID-19, as well as T-COVID, which is an immune modulator for treating the early stages of COVID-19.
Source: Corporate presentation
COVID-19 Treatments
AdCOVID, which is the company's vaccine, is due to begin a phase 1 trial in December 2020. This vaccine has several characteristics that appear to be superior to the leading, first generation, vaccines (I will refer to the first generation vaccines in the rest of the article as "G-1" or "G-1 vaccines"). These characteristics include:
- Single-dose vaccine (to be fair, so is the J&J (NYSE:JNJ)vaccine).
- Administered through a nasal spray rather than an injection.
- Can be distributed with no cooling. Can be stored for months in room temperature.
T-COVID is aimed at treating early cases of COVID-19. Like AdCOVID, it is also an intranasal single-dose therapeutic. T-COVID phase 1/2 trial is ongoing with 96 confirmed COVID-19 patients randomized 1:1 to T-COVID or placebo. The trial's primary endpoint is clinical worsening, defined as a decrease in oxygen saturation or hospitalization. I'm not sure the size of the population here is powered to generate statistically significant results, but hopefully, we should be able to see a directional result. Data readout of the ongoing trial is expected for Q1 2021.